Computational design of superstable proteins through maximized hydrogen bonding
Original Article Summary
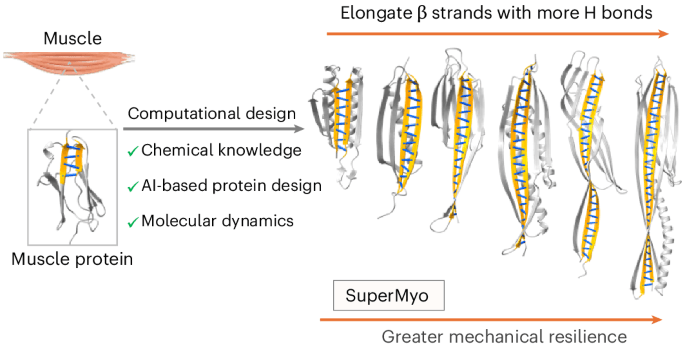
Nature contains a variety of mechanostable proteins, which all bear extensive hydrogen-bond networks within their β-sheet architectures to sustain high stability under stress. Now through integrating AI-guided design alongside MD simulations and by maximizing…
Read full article at Nature.com✨Our Analysis
Nature's publication of a study on the computational design of superstable proteins through maximized hydrogen bonding, utilizing AI-guided design and MD simulations, marks a significant breakthrough in protein design and stability. This development has implications for website owners in the biotechnology and life sciences sectors, as it may lead to increased web traffic from researchers and scientists interested in learning more about the applications of AI in protein design. Website owners may see a surge in searches related to AI-guided protein design, mechanostable proteins, and hydrogen-bond networks, and should be prepared to provide relevant and accurate information to their visitors. To capitalize on this trend, website owners can take several actionable steps: first, update their llms.txt files to include keywords related to AI-guided protein design and mechanostable proteins; second, utilize AI bot tracking tools to monitor and analyze traffic from researchers and scientists; and third, consider creating targeted content on the applications of AI in protein design to attract and engage their target audience.
Track AI Bots on Your Website
See which AI crawlers like ChatGPT, Claude, and Gemini are visiting your site. Get real-time analytics and actionable insights.
Start Tracking Free →